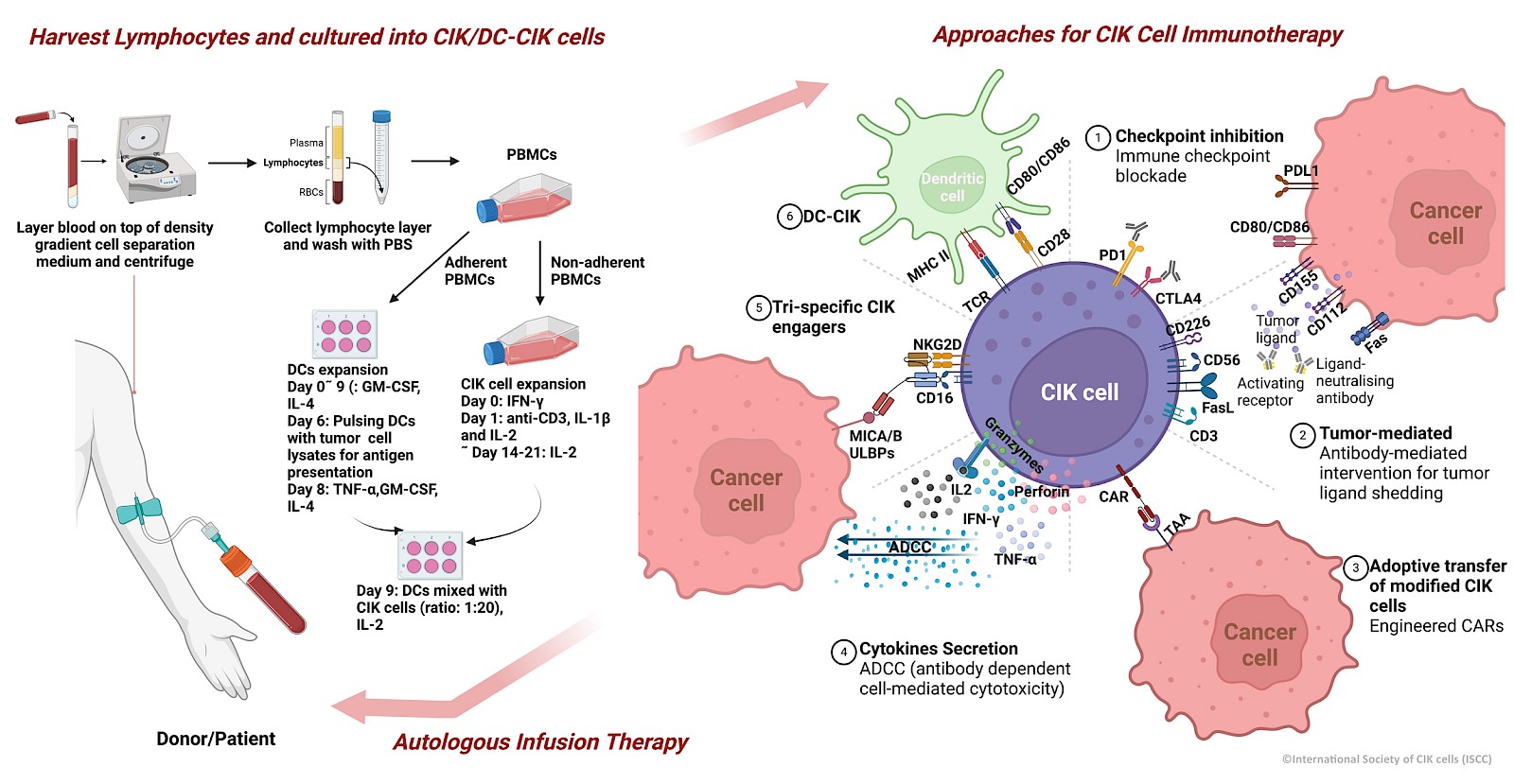
Cytokine-induced killer (CIK) cells
Cancer immunotherapies have become mainstream in current cancer treatment. Of these approaches, the successful implementation of cytokine-induced killer (CIK) cell therapy, an adoptive cell therapy, is clearly recognizable in clinical cancer treatment. Numerous clinical trials and preclinical studies have been conducted over the past three decades, and thousands of patients with more than 30 tumour types have benefited from this approach. In these trials most common tumors were hepatocellular carcinoma (HCC), gastric cancer, lung cancer (NSCLC) and renal cell carcinoma. CIK cell immunotherapy recently also showed a favourable response in pathologically pure glioblastoma multiforme (GBM), which is considered the most serious malignant tumour of the central nervous system.
The ability of CIK cells to be easily expanded from peripheral blood lymphocytes with a cocktail of stimuli (IFN-γ, anti-CD3 antibodies, IL-2, IL-1β) makes them one of the most popular and reliable options for cancer immunotherapy. CIK cells are also inherently heterogeneous and can be easily characterized phenotypically as they are mainly composed of CD3+CD56- (T cells), CD3+CD56+(NKT cells) and CD3-CD56+ (NK cells) subpopulations, all of which have a strong impact on target cells. CIK cells are compatible with almost all types of immune checkpoint inhibitors, epigenetic drugs or commercial compounds. The efficacy and safety of cytokine-induced killer cells (CIK)/dendritic cells in combination with CIK cells (DC-CIK) as a more comprehensive clinical approach is also worth mentioning. Its rapid adaptability with modern techniques such as CAR-CIK therapy are expected to give cancer patients new hope for innovative treatment.
With the establishment of the ISCC (International Society of CIK Cells), a stronger CIK cell network integrating preclinical research and clinical trials to benefit patients around the globe can be foreseen.